
|
|
OCTOBER 2023
|
|
|
IN THIS NEWSLETTER:
- From the Cancer Consortium
- CCSG Updates
- In the Spotlight
- Consortium Kudos
- Assistance is Available through the Research Development Office
- Current Funding Opportunities
- Save the Date - Upcoming Events
- From the NCI
- From the Office of Faculty Affairs & Diversity
- From the Integrated Research Centers (IRCs)
- From the Office of Community Outreach & Engagement
- From the Consortium Shared Resources
- Team Spotlight: Get to Know the OCOE Patient Navigator Team!
|
|
|
|
|
|
|
FROM THE CANCER CONSORTIUM
|
CCSG Updates
Revision, revision, and more revision! As our submission deadline of January 25, 2024, draws closer, Consortium leadership is working hard on editing and refining the final drafts of their narratives for the CCSG, which are due at the beginning of November.
Meanwhile, the Consortium administration team is in the process of compiling and formatting supplementary materials for the grant, including membership rosters, funding data, letters of support, and more.
|
In the Spotlight
The following inter-institutional Cancer Consortium collaboration was featured in the most recent edition of the Science Spotlight:
|
Consortium Kudos
In addition to cooler weather, October brought lots of exciting news to celebrate! Please join us in congratulating the following Consortium members on their accomplishments:
|
|
|
|
- Congratulations to Drs. Pete Nelson (PC), Dan Lin (PC), Colm Morrissey (PC), and the rest of the Prostate Cancer SPORE team for successfully renewing the Pacific Northwest Prostate Cancer SPORE on September 8! This SPORE proposal enlarges the blueprint for a well-built, distinctive and coordinated translational prostate cancer research effort spanning the entire PNW. It also includes a proposal for a Developmental Research Program and a Career Enhancement Program that will substantially strengthen the translational goals of the prostate cancer research program and expand opportunities for engaging young as well as established investigators in our multidisciplinary environment.
|
|
|
|
- Congratulations to Drs. Liz Swisher (BOC), Barbara Norquist (BOC), and more for the publication of Making Genetic Testing Accessible (MAGENTA), a randomized controlled trial sponsored by Stand Up to Cancer. You can read the full article, "Remotely Delivered Cancer Genetic Testing in the Making Genetic Testing Accessible (MAGENTA) Trial" in JAMA Oncology.
- Congratulations to Dr. Chris Kemp (CBB) and his team for receiving notice of their third successful U01 grant for their project, "A Patient-Centric Approach to Advance Functional Precision Oncology," from the NCI's Office of Cancer Genomics, Cancer Target Discovery and Development (CTD2) Network. CTD2 is a functional genomics initiative that bridges the gap between genomics and development of effective therapeutics. Only seven centers were funded for the current cycle, and Fred Hutch is one of them. In addition to Dr. Kemp, who serves as the PI, this project also involves Consortium members Drs. Behnam Nabet (CBB) and Liz Swisher (BOC).
|
Assistance with Large Collaborative Grants Is Available through the Research Development Office
The Consortium's Research Development Office (RDO) offers support for large collaborative grants, including (but not limited to) SPOREs, U54s, and P01s. The RDO works one-on-one with Consortium members to connect them with relevant resources and provide tailored support, from project management to editing and formatting.
|
|
|
|
|
|
|
CURRENT FUNDING OPPORTUNITIES
|
NCI Administrative Supplement
The National Cancer Institute (NCI) has supplement funding available through the Early Career Cancer Clinical Investigator Award (ECIA) that recognizes and supports outstanding early career clinical investigators who demonstrate a commitment to becoming an academic clinical researcher and supporting their cancer center’s NCI-funded clinical trials enterprise. The ECIA is designed to promote the retention of early career investigators who wish to initiate a career path that focuses on interventional cancer clinical trials and academic clinical research.
Eligibility Criteria:
Please review the RFA (found here) for full eligibility criteria.
- The candidate must not be the PI of a National Institutes of Health (NIH) peer-reviewed research grant of at least $125,000 in direct costs per year for a minimum of 3 years, with the exception of mentored career development awards or awards where the PI is required to be mentored by another investigator; or the PI of a peer-reviewed research grant of at least $125,000 in direct costs per year for a minimum of 3 years from any of the organizations listed in the RFA.
- The candidate must be one of the following: Physician (e.g., M.D., D.O.), board certified or have equivalent training and qualifications in specialty area, e.g., medical oncology, radiation oncology, surgical oncology, gynecologic oncology, or equivalent; Oncology nurse or clinical psychologist (or similarly qualified clinician) with a doctoral degree.
- The candidate must be a full-time faculty member, eligible for promotion, and at least two years post last clinical fellowship and no more than six years from initial academic appointment at the time of the application submission deadline of December 18, 2023 (including appointments held at other institutes).
- The candidate must have potential for leadership of the Cancer Center’s clinical trials infrastructure activities. Involvement in the cancer center clinical trial related activities (e.g., protocol review committees, Institutional Review Board, etc.) is encouraged but not required at the time of the award; however, it is expected that ECIA recipients will be appointed to at least one of these committees during the award period.
- Involvement in NCI clinical trial networks (NCTN, NCORP, ETCTN, CITN, etc.) and or NCI Scientific Steering Committee/Task Force activities is encouraged but not required at the time of the award; however, it is expected that the ECIA recipients will be involved in at least one of these activities during the award period.
The FH/UW/SC Cancer Consortium can submit one application to this funding opportunity. All interested candidates should send a one-page letter of interest that includes the candidate’s suitability for the award, a half-page description of planned activities, and an attestation that the candidate meets the full eligibility criteria to cancerconsortium@fredhutch.org by Friday, October 13th, 2023. One applicant will be selected by Consortium leadership to submit an application for the December 18th, 2023 submission deadline.
Please direct any questions to Kris Blair (kblair@fredhutch.org), Research Development Specialist, Cancer Consortium Administration.
|
|
|
|
|
|
|
UPCOMING EVENTS
|
» October 16, 2023: Discover S8 Learning Day Hosted by the Consortium Shared Resources
To help facilitate a smoother launch of the Flow Cytometry Core's new BD FACSDiscover S8 with CellViewTM Cell Sorter (Discover S8), we are partnering with BD to bring a Discover S8 Learning Day to Fred Hutch. This full day symposium will take place on Monday, October 16th, in the Pelton Auditorium (Fred Hutch Campus). More details to follow; please be on the lookout for a registration email.
For more information about the Discover S8, please see the "From the Consortium Shared Resources" section below.
|
» November 3, 2023: Joint Breast & Ovary Cancers and Prostate Cancer Program Retreat
Please join the Breast & Ovary Cancers and Prostate Cancer Programs for a joint retreat. The retreat, titled "Hormones in Cancer," will take place on Friday, November 3, from 8am-1pm in the O'Mack Symposium Suites (Steam Plant Building, Fred Hutch Campus).
Stay tuned for an agenda and more details in the coming weeks!
Priority registration for this retreat will be given to members of the BOC and PC programs. Other Consortium members are welcome to attend as space allows.
|
|
|
|
|
|
|
FROM THE NCI
|
The NCI-Sponsored ORBIT Institute: Developing Behavioral Treatments to Improve Health is now accepting applications for 2024!
Please note the application portal will close on November 30, 2023.
This course will be open to scientists with an interest in behavioral treatment development to improve health behaviors. While applied behavioral and social scientists are the focus, basic scientists and methods experts are encouraged to apply as well. Any post-graduate investigator (doctoral or terminal degree received) in the medical, behavioral, social, and statistical/methodology sciences who has a demonstrated, pre-existing interest in contributing to investigator teams in developing and testing behavioral treatments is eligible to apply to this course. Please visit our FAQ page and if you have additional questions, you may contact the ORBIT team at orbit.institute@med.fsu.edu or 850-644-2334.
If your application is accepted, you will be required to:
- Attend the in-person ORBIT Institute Workshop in Tallahassee, FL, on May 7-10, 2024 (non-negotiable)
- Attend bi-weekly webinars (i.e. virtual workshop training seminars) following the in-person meeting
|
|
|
|
|
|
|
FROM THE OFFICE OF FACULTY AFFAIRS & DIVERSITY
|
Applications Are Open for the President's Postdoctoral Fellowship Program
The President’s Postdoctoral Fellowship Program was established by the University of California in 1984 as way to encourage women and minority Ph.D. recipients to pursue academic careers. In 2023, Fred Hutchinson Cancer Center joined a collaborative partnership with the University of California to offer postdoctoral fellowship opportunities, joining other members such as UNC at Chapel Hill, University of Michigan, Georgia Tech, and more. The current program provides mentoring, professional development, and academic networking opportunities to fellows. PI’s currently hiring postdocs are encouraged to have eligible candidates apply to the program, with the expectation that the PI will serve as their mentor. Applications are now open until Nov 1, 2023.
|
|
|
|
|
|
|
FROM THE INTEGRATED RESEARCH CENTERS (IRCs)
|
The IRCs are Hiring!
Please see the open positions below and consider sharing them with your network:
Immunotherapy IRC Physician Scientist Program
The Immunotherapy IRC’s Physician Scientist Program is still accepting applications. This is a great opportunity to provide two years of mentorship, training, and financial support to an MD or MD/PhD who seeks to advance their career in immuno-oncology research. Current recipients of this program are Lorenzo Iovino, MD, PhD, in the Dudakov Lab, and Francesco Mazziotta, MD, PhD, in the Chapuis Lab. CRD Assistant Professor Jordan Gauthier, MD, MSc and CRD Research Associate Alexandre Hirayama, MD, are past recipients.
For more information and to apply for this opportunity, click here. We are accepting applications on a rolling basis until the position is filled.
Pathogen Associated Malignancies IRC Open Faculty Position
Fred Hutchinson Cancer Center invites applications for an open faculty position at the Assistant or Associate Professor level in the Pathogen Associated Malignancies Integrated Research Center. We seek a laboratory-based scientist working in the area of cancer caused by pathogens, or in the role of the microbiome in cancer. This person will have a primary appointment in the Human Biology Division and share its goals to advance an understanding of the biological basis of cancer and to translate findings to the clinic.
For more information or to apply for this position, click here.
|
|
|
|
|
|
|
FROM THE OFFICE OF COMMUNITY OUTREACH & ENGAGEMENT
|
Comprehensive Cancer Control Plan Feedback Successfully Submitted!
Thank you to everyone who read and provided feedback on the draft of the Comprehensive Cancer Control Plan. Over the last few weeks, we've reviewed and compiled your feedback into a single report. This report was signed by Dr. Lynch and submitted to the Washington State Department of Health.
Updating the state's Comprehensive Cancer Control Plan is an incredibly important step toward a healthier future, and the feedback provided by the Cancer Consortium will be vital in refining the Plan. We appreciate your help with this important public health effort!
|
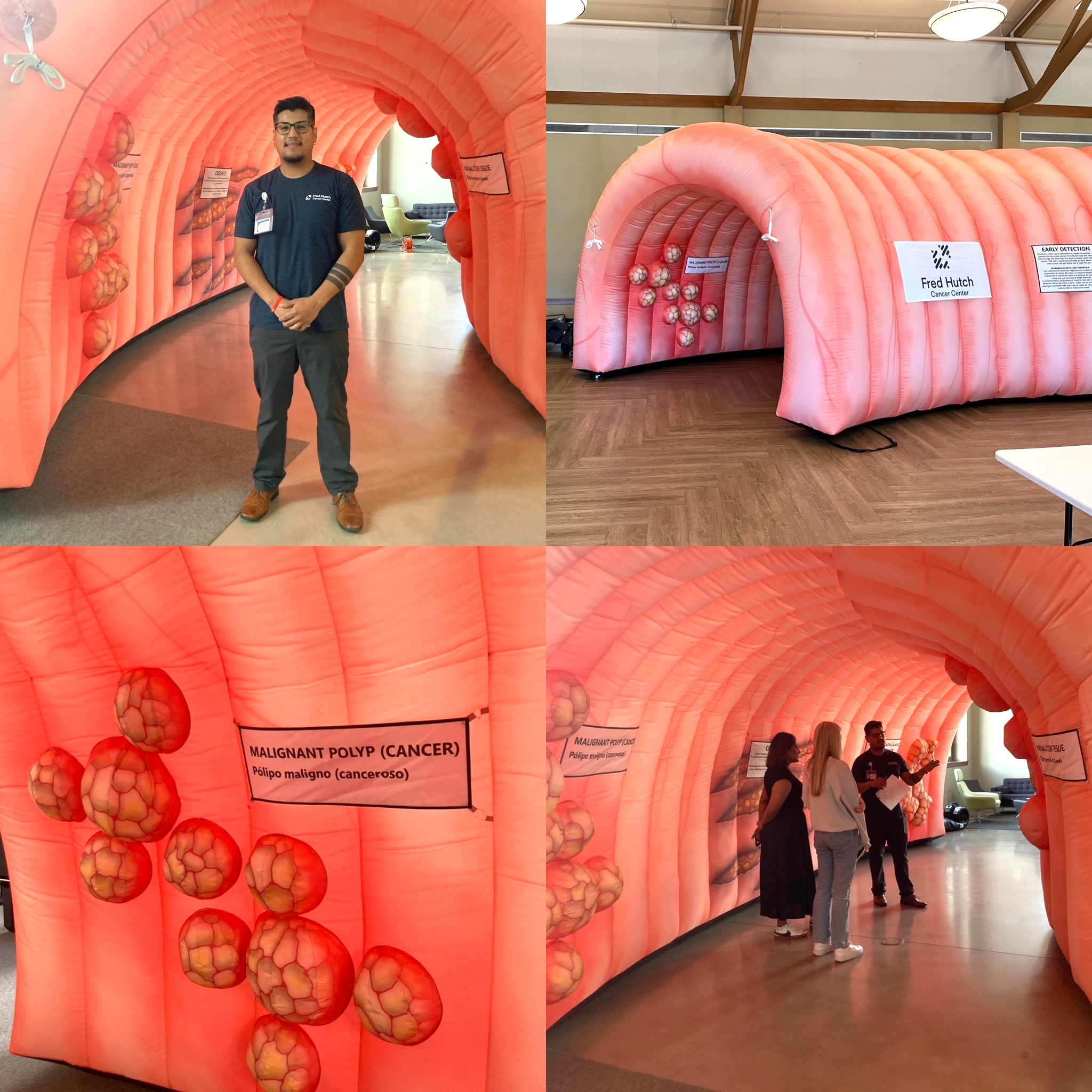
Meet CRAIG the Colon!
We are excited to announce that after holding a community naming contest, Luanne in Spokane had the winning entry for our inflatable colon in Eastern Washington! Meet CRAIG: ColoRectal Abnormalities In the Gut. If you or any of your community partners are interested in having an inflatable colon at a community event to raise awareness about colorectal cancer and the importance of screening for early detection, let us know! We would love to partner with you to bring out CRAIG in Eastern WA, CASPER in Central WA, or CECE in Western WA. Learn more about the inflatable colon specs here or submit a collaboration request form here.
|
|
|
|
|
Community-Researcher Connections
OCOE is now including a space in their monthly newsletter to offer opportunities to connect researchers with community members. Check out the September newsletter. Looking for community members to help guide or advise a research study? Need to get the word out to the community about an opportunity to participate in research? Let us know at EndDisparities@fredhutch.org and we’ll include in the next issue!
|
|
|
|
FROM THE CONSORTIUM SHARED RESOURCES
|
New FACSDiscover S8 CellView
We are excited to announce that the Flow Cytometry Shared Resource (FC-SR) received Fred Hutch capital equipment funding for the purchase of the first-of-its-kind spectral imaging sorter, the BD FACSDiscover S8 with CellViewTM Cell Sorter (Discover S8). We recently learned that PACCAR Inc. generously provided funding to cover the cost of the Discover S8. This is an amazing gift to the Center. The FC-SR relies mainly on capital equipment funding for the purchase of instrumentation and those funds can now be used for other high-value items to support the Center's research needs.
|
|
|
|
The Discover S8 will be replacing the Aria II-2 and will be housed in the Thomas lab in DE-766. The S8 has arrived, and installation and training are underway. This is expected to take 1-2 weeks. The instrument will be added to the iLab calendar when it is open for users to begin training and using the system.
The Discover S8 has new features, including a full spectral system with a full array of 78 detectors to capture emission spectra from 350nm to 850nm. The system integrates six real-time imaging detectors with three fluorescent and three scatter parameters. Because the system integrates images with traditional and spectral cytometry, there are new workflows and panel design considerations that will be critical to successful experiments for those that want to take full advantage of the imaging and spectral capabilities.
|
"When first using the S8, I demo-ed one of our lab's 28 c panels on the machine first designed for an A5 Symphony. There were obvious improvements in the resolution of the panel due to being collected on a full-spectrum instrument that was pre-optimized. The user interface was of high quality and essentially allowed me to operate the machine right away due to the step-by-step prompts. On the imaging side, I was very impressed that the software generates a metric of co-localization (along with many other imaging metrics) from the imaging detectors so that this information can be immediately analyzed alongside the full spectrum detectors."
-Andrew Konecny, Prlic Lab
|
|
|
|
|
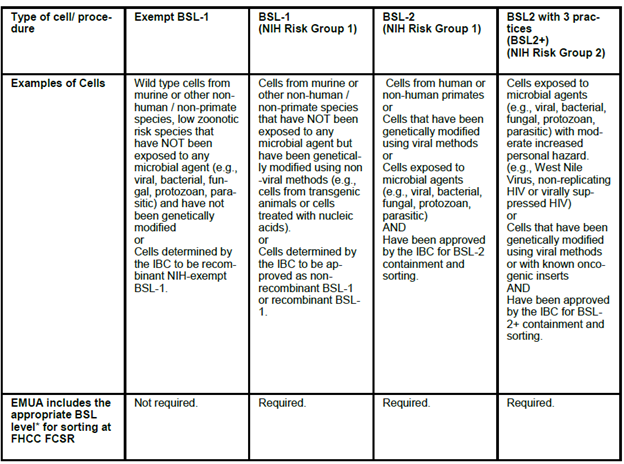
Biosafety and Cell Sorting
|

|
To address this issue, a long-overdue upgrade to house the cell sorters in Class IIA biosafety cabinets is finally complete and the flow cytometry lab now has an approved EMUA to allow sorting of BSL-1, BSL-2, and BSL-2+ designated samples that are approved by the Institutional Biosafety Committee (IBC) and EH&S. There will be several steps that need to be completed before researchers will be granted approval to use the sorter for samples in Risk Group 2 (RG2).
|
|
|
|
Cell sorting increases the risk for aerosolization of potentially hazardous agents. Due to the lack of full aerosol containment, the sorters in the flow cytometry lab were all designated as Biosafety level 1(BSL-1) for live cell sorting. This has been a hindrance for researchers and did not reflect the reality of what has been historically sorted in the lab.
|
|
|
|
- All users will need to fill out the Sorting Approval Form. The form will ask for the lab EMUA #, the cell origins and any agents the cells have been exposed to, providing transparency and to determine what can be transported and sorted in the flow lab. Cells infected with RG2 agents and RG2 bacteria and yeasts, which have an increased potential for infection through a respiratory or mucosal route, will require prior approval from the Institutional Biosafety Committee (IBC) and EH&S. These agents must be described on the current EMUA or added with an amendment to the PI’s EMUA to include live cell sorting and have the appropriate BSL designation. Samples that will need to be sorted as BSL-2+ will require additional training from the flow cytometry staff. Samples that are designated as Risk Group 3 cannot be sorted in the lab without a validated fixation method.
- We will require at least one member of each lab to upload the lab’s EMUA for our records. Each subsequent lab member can reference that EMUA #.
- The next step will be rolled out once our frequent users begin to fill out the Sorting Approval Form, have their approved EMUA on file and receive training. Training will include reading the Flow Cytometry Cell Sorting Biosafety SOP, provided once the approval form is complete, and hands-on instruction with the flow cytometry staff. Users will need to have completed the training and be approved to schedule sorting time. The iLab scheduling calendar will include a sample information form that will need to be completed for each sort, including which cells and agents will be transported and sorted in the lab.
When users arrive for their sorts, there will be a donning station outside of the sorter rooms. The lab will provide the necessary PPE for each BSL; users are welcome to bring their own if what is supplied doesn’t work for them.
Since this is a new process, there may be some hiccups as the staff and users start to use the new system. Please be patient with us and if you have any concerns or questions, please contact Michele Black ( mblack@fredhutch.org) or the lab email ( flowcytometry@fredhutch.org).
See the table for general information about risk group and BSL designation. Contact EH&S for questions about your samples risk group if you are unsure.
|
|
New Data Management System
The Flow Cytometry lab has been working to better manage the data generated within the lab. Historically, data has been archived on shared network drives like Fred Home, HomeLink and Fast in our flow cytometry lab share, organized by dated folders for each instrument. This has made locating data challenging, especially when the researcher is trying to find old data from a collaborator, departed lab mate, or even their own data that they have lost. If they only have a rough estimate of when it was created, it is a laborious process for the staff to hunt through folders to retrieve these data files. Additionally, there was not a standardized way of exporting the data from the analysis software, with users directly exporting to portable drives, connecting to shared drives, to Google and so on. Simply put, it has been a free-for-all and a data management headache.
The first step in getting a robust system in place was to ensure data from the lab is saved and organized. We have created user logins on most cytometers to automate data export to user specific folders; Sony sorters and ID7000 are the exceptions. This now makes sure everyone's data is in a centralized location organized into shared lab folders, with each member having their own folder containing their experiments and data files. Currently, users are still copying data to their own storage preference; we will phase out the option of using any portable devices (hard drives or thumb drives) as these are a security risk. Users who still want to save a copy to their own storage media, will be able to download from Cirro using a web browser. Now that the first step is complete, our next step is to upload all recent data to a storage gateway that will then be ingested into Cirro. Cirro was developed by the Hutch Data Core and will eventually be utilized by many of the Centers’ shared resources.
|
|
|
|
“Cirro simplifies access to cutting-edge cloud technologies and addresses common research challenges of working with large-scale 'omics datasets — from data ingestion, management, and analysis to ad hoc exploration, visualization, and publication.”
|
|
|
|
As we complete the next phase of the project, we will be helping users access data from Cirro. Check out Cirro for detailed information and be on the lookout for training opportunities with flow cytometry and the Hutch Data Core.
|
|
New Lago X Installed in Comparative Medicine and Preclinical Imaging Shared Resources
|
|
|
|
Preclinical Imaging and Comparative Medicine Cores have a new bioluminescence, fluorescence and X-ray imaging system – the Lago X – which is installed in the Day campus vivarium. This instrument is fully operational and ready for use! If you or anyone you know are interested in using this instrument, please reach out to preclinicalimaging@fredhutch.org.
|
|
|
|
|
|
|
GET TO KNOW THE OCOE PATIENT NAVIGATOR TEAM
|
Patient navigators are an essential resource for those undergoing cancer treatment. They connect cancer patients and their families/caregivers with services like transportation and lodging, culturally sensitive support, community resources, and more. They also help patients navigate the complexities of the healthcare and insurance systems.
This month, we polled the OCOE patient navigator team about their favorite parts of their jobs, their most meaningful takeaways, and - of course - their hot takes on Spooky Season!
In this poll:
- Anne Devine, Manager, Patient Navigation (FH South Lake Union Campus)
- Chloe Lunn-Fisher, Clinical Patient Navigator (FH South Lake Union Campus)
- Shavon Yingling, Patient Navigator (FH South Lake Union Campus)
- Lynne O'Keefe, Clinical Patient Navigator ((FH South Lake Union Campus)
|
What's your favorite part of your job?
- Anne: Working with patients and caregivers and our team members.
- Chloe: Finding new resources and creative ways to help patients overcome barriers to care.
- Shavon: My favorite part of the job is connecting with the different patients and families and being able to connect them to different resources that can help take away some of the stress or ease a burden so they can spend their time focusing on the things that matter most.
- Lynne: Supporting patients who are most in need.
|
What is the most important or impactful thing you've learned about patient navigation?
- Anne: A deeper understanding of institutional racism and inequity in health care. I was aware before yet also had more blinders about it.
- Chloe: Patient Navigation has the power to improve health care outcomes.
- Shavon: This is a hard question. I feel I learn something new about patient navigation every day. I've learned the outcomes may not always be what we want for the patients we advocate for, but I've realized there's so much value in just being a supportive advocate for the patient and sometimes just a listening ear.
- Lynne: Some patients just want to be heard.
|
Describe your most memorable Halloween costume.
- Anne: Space Queen.
- Chloe: I dressed up as Medusa and glued a bunch of plastic snakes into a wig - it ended up being SO heavy I could barely keep my head up!
- Shavon: Nothing super memorable, but definitely fun! I loved dressing up as the Cat In the Hat!
- Lynne: Smurfette as a kid.
|
What is your favorite candy?
- Anne: Salted caramel chocolate.
- Chloe: Anything sour-gummy.
- Shavon: Give me chocolate! Snickers, Reese's, Butterfingers... Yum!
- Lynne: Reese's peanut butter cups.
|
What's your favorite scary movie?
- Anne: The Birds.
- Chloe: I'm not a big scary movie person but my favorite movie to watch this time of year is Young Frankenstein.
- Shavon: The Strangers.
- Lynne: Knives Out.
|
What if your ideal way of celebrating Halloween?
- Anne: Staying home and watching a good movie.
- Chloe: Staying in and watching movies, and hoping to get a least one trick-or-treater!
- Shavon: Passing out candy to the kids while watching scary movies at home. It'll happen one day! For now, I'm still out there with the rest of the parents escorting my munchkin around.
- Lynne: With friends and family.
|
|
|
FRED HUTCH/UNIVERISTY OF WASHINGTON/SEATTLE CHILDREN'S CANCER CONSORTIUM
1100 FAIRVIEW AVE. N., SEATTLE, WA 98109
Award number P30 CA015704-48
|
|
|
|
|